Abstract
Background: Achieving undetectable MRD has become an endpoint in MM. However, there is a considerable fraction of patients (pts) with positive MRD after initial therapy. Emerging data suggests that prolonged treatment using the same drugs does not abrogate the poor prognosis of persistent MRD. Because these cells embody the notion of ultra-drug resistance, it could be hypothesized that a better understanding of MRD biology is needed to eradicate it and improve outcomes in MM.
Aim: Understand MRD resistance through multiomics profiling of patient-paired tumor cells at diagnosis, MRD and relapse after different treatment modalities.
Methods: This study included 118 pts, 28 elderly and 90 transplant-eligible. The former received lenalidomide and dexamethasone (Rd) +/- clarithromycin until disease progression (GEM CLARIDEX). The latter included high-risk smoldering (n = 6) and active MM (n = 84), respectively enrolled in the GEM CESAR and GEM2012MENOS65 trials. Both consisted in induction (KRD/VRD, respectively), followed by autologous transplant (HDT/ASCT) and consolidation (KRD/VRD) prior maintenance.
A total of 289 bone marrow samples were analyzed at diagnosis (n = 118), after induction (n = 96), HDT/ASCT (n = 34), consolidation (n = 13) and relapse (n = 28). Next generation flow cytometry was used to identify patient-specific aberrant phenotypes prior isolation of tumor cells using FACS. Co-isolation of RNA and DNA was performed prior RNA and whole-exome sequencing (WES). Differential expression was performed using DESeq2. Peripheral blood T cells were used for germline control.
Results: Transcriptional analysis of patient-paired tumor cells at diagnosis vs persistent MRD after Rd in elderly MM and KRD/VRD induction in transplant-eligible pts, revealed differential expression of only 11 and 15 genes, respectively. By contrast, there were 646 differentially expressed genes between diagnosis and persistent MRD after HDT/ASCT, and 252 after consolidation. Genes commonly deregulated in MRD post HDT/ASCT and consolidation vs tumor cells at diagnosis in pts who relapsed, were enriched in biological processes involving cell activation in immune response.
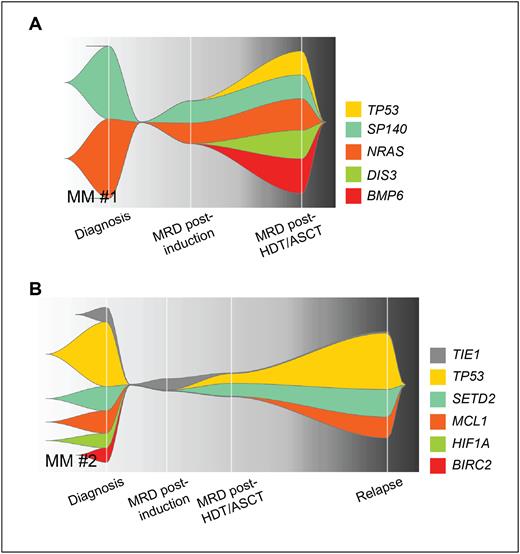
It was interesting to note that only 6 genes were commonly upregulated in MRD at all timepoints. Therefore, transcriptional findings suggested that clonal evolution and/or selection peaked after HDT/ASCT. To confirm this hypothesis, the genomic traits of MRD resistance were subsequently investigated using WES in 28 pts with paired tumor cells isolated at diagnosis and MRD at multiple timepoints (n = 63 samples). Comparable with RNAseq, there was a median of 36% (3 - 68) mutations shared between paired tumor cells at diagnosis and MRD post induction, while only 4% (0 - 51) were shared between diagnosis and MRD after HDT/ASCT, and 7% (4 - 58) after consolidation. Most unshared mutations appeared de novo in MRD cells. As an interesting example, pt1 presented de novo TP53 mutation post HDT/ASCT, never achieved undetectable MRD with consolidation and maintenance, and relapsed soon after (Fig 1A).
Being persistent MRD intrinsically associated with progressive disease, we next compared the transcriptional and genomic profiles of patient-paired tumor cells at diagnosis, MRD and relapse. Unsupervised analysis showed that tumor cells at relapse clustered closely with those at diagnosis rather than MRD clones, with 3 and 23 differentially expressed genes observed, respectively. Genomic analyses revealed a median of 11% (1 - 49) and 6% (1 - 65) shared mutations between tumor cells at relapse compared to diagnosis and MRD, respectively. As another interesting example, pt2 had mutated TP53 that became undetectable post induction but reemerged in MRD cells after HDT/ASCT and finally became dominant at relapse (Fig 1B).
Conclusions: We present the largest dataset of longitudinal MRD profiling in MM. Our results reveal transcriptional stability during induction with relative divergence after HDT/ASCT, as well as progressive genomic evolution that peaked after HDT/ASCT. These findings could help explain the continued responses with longer induction regimens, and limited efficacy of consolidation after HDT/ASCT observed in recent studies. Furthermore, the transcriptional and genomic singularity of MRD cells when compared to diagnosis and relapse, suggests that using drugs with alternative modes of action are needed to overcome MRD resistance.
Disclosures
Puig:Celgene: Honoraria, Speakers Bureau; Amgen, Celgene, Takeda and The Binding Site: Honoraria; Celgene, Janssen, Amgen andTakeda: Research Funding; Amgen, Celgene, Janssen and Takeda: Consultancy. Garcia:Janssen, Celgene (BM), Takeda: Consultancy; Janssen ,Celegene (BM), Takeda, GSK , Sanofi, Amgen: Honoraria, Speakers Bureau. Oriol:Sanofi: Consultancy, Speakers Bureau; Janssen: Consultancy; GlaxoSmithKline: Consultancy, Speakers Bureau; Bristol Myers Squibb: Consultancy, Speakers Bureau. Rios:Becton-Dickinson, Celgene, GSK, Janssen, Sanofi, Binding Site: Consultancy. De Arriba:Amgen: Consultancy, Honoraria, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Speakers Bureau; Glaxo Smith Kline: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau. Arguiñano:Novartis: Honoraria; Sandoz: Speakers Bureau; Amgen: Honoraria; Janssen: Honoraria; BMS-Celgene: Honoraria; GSK: Honoraria; AstraZeneca: Honoraria; Sanofi: Honoraria; Abbvie: Honoraria. Bladé Creixenti:Janssen, Calegen/BMS,Amgen, Takeda, Oncopeptides: Honoraria. Mateos:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. San-Miguel:Abbvie, Amgen, BMS, Celgene, GSK, Haemalogix, Janssen-Cilag, Karyopharm, MSD, Novartis, Takeda, Regeneron, Roche, Sanofi, and SecuraBio: Consultancy, Other: Advisory Board. Paiva:Janssen: Consultancy, Honoraria; EngMab: Research Funding; GSK: Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Amgen: Honoraria; Adaptive: Honoraria; Takeda: Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Roche Glycart AG: Honoraria, Research Funding; Gliead: Honoraria; Oncopeptides: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal